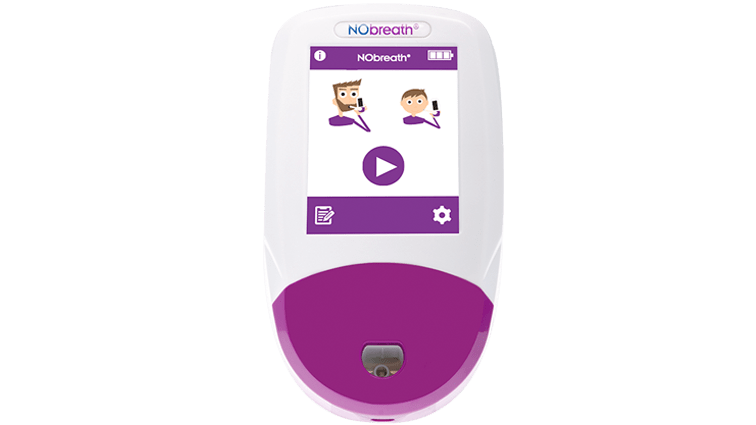
The NObreath® has been a proven technology for over ten years of clinical use in studies worldwide.
The NObreath® is a FeNO (fractional exhaled nitric oxide) monitor which can be used to measure airway inflammation for the management and diagnosis of asthma. The NObreath® makes FeNO monitoring, which is recommended by ATS, quick and easy plus it is completely non-invasive, with the ability to monitor airway inflammation in both adult and child patients.