Description
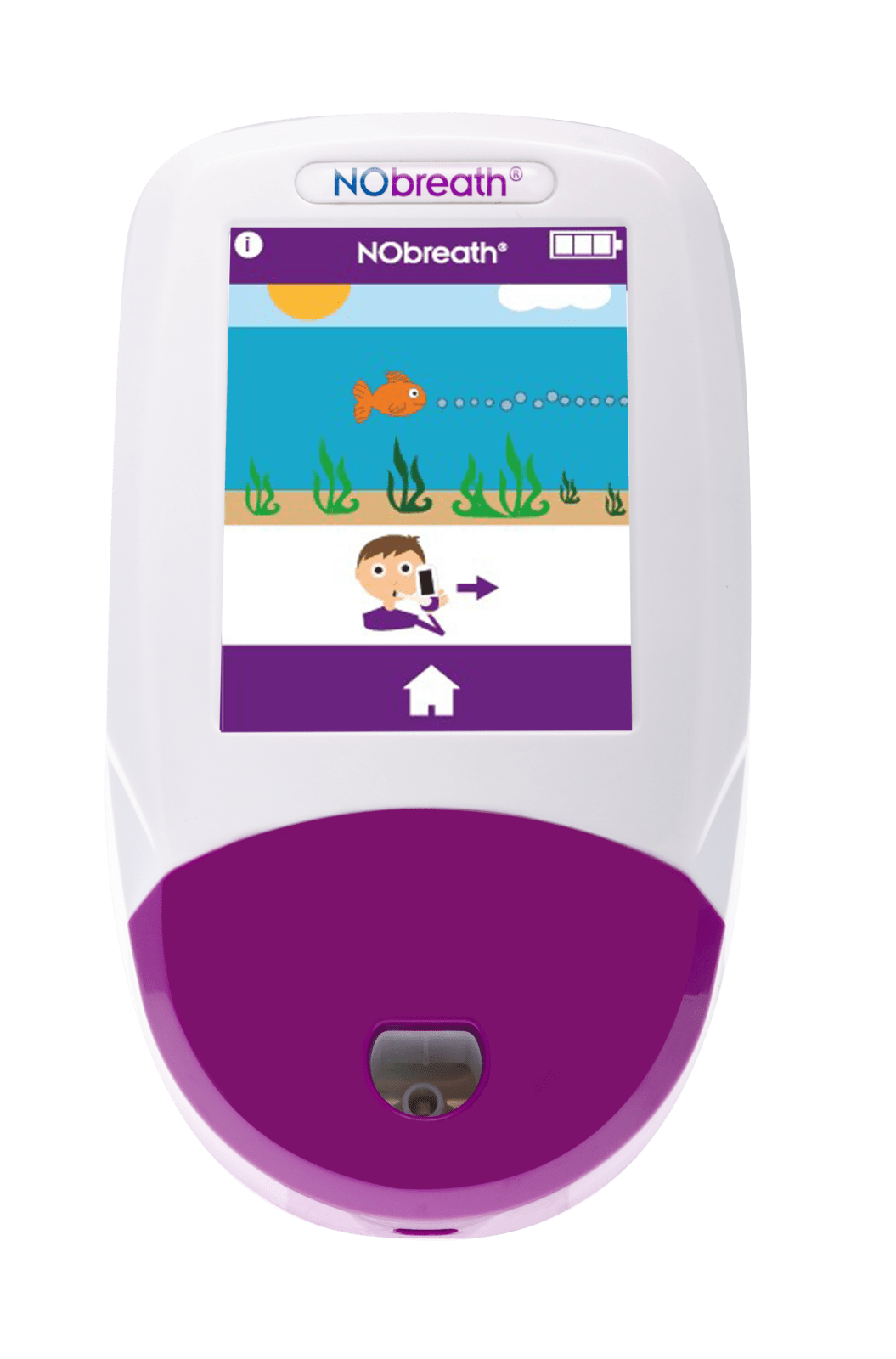
NObreath®
FeNO testing without limits™
ONE BREATH AT A TIME

The NObreath® has been a proven technology for over ten years of clinical use in studies worldwide.
The NObreath® is a FeNO (fractional exhaled nitric oxide) monitor which can be used to measure airway inflammation for the management and diagnosis of asthma. The NObreath® makes FeNO monitoring, which is recommended by The American Thoracic Society (ATS), quick and easy plus it is completely non-invasive, with the ability to monitor airway inflammation in both adult and child patients. Furthermore, the ambient monitoring mode enables you to check ambient levels of NO (nitric oxide).

Key Features
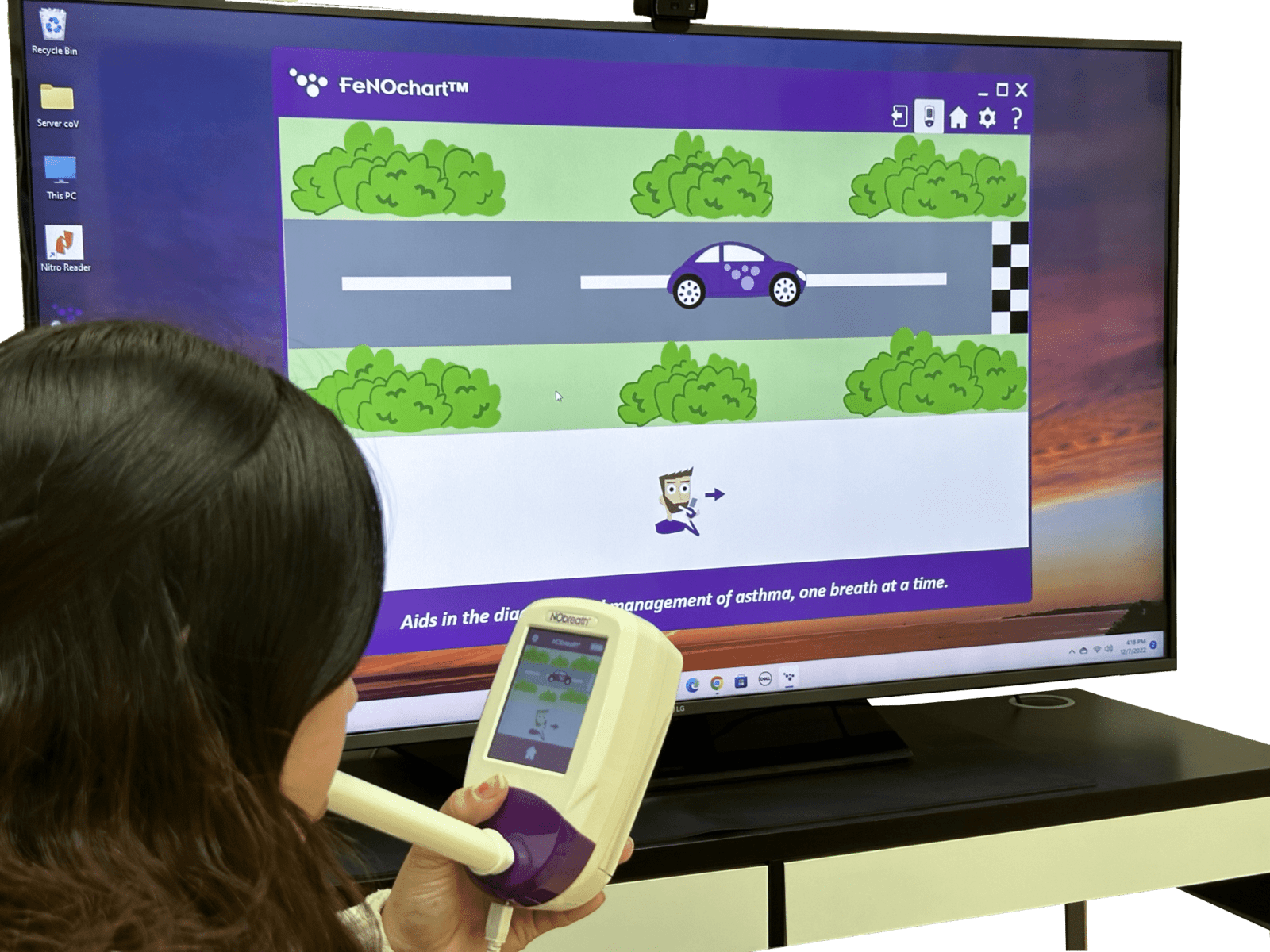
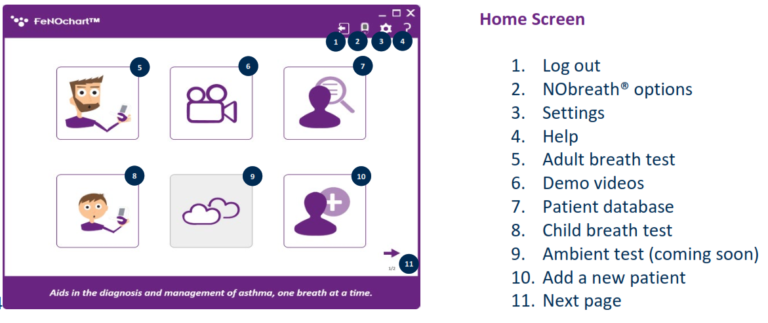
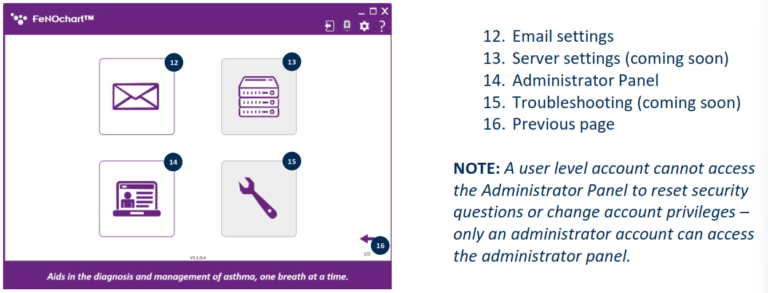
NObreath® FeNOchart™ Software Beta
• GDPR Compliant
• Create, store, and manage patient profiles
• Adult and child breath tests with screen mirroring
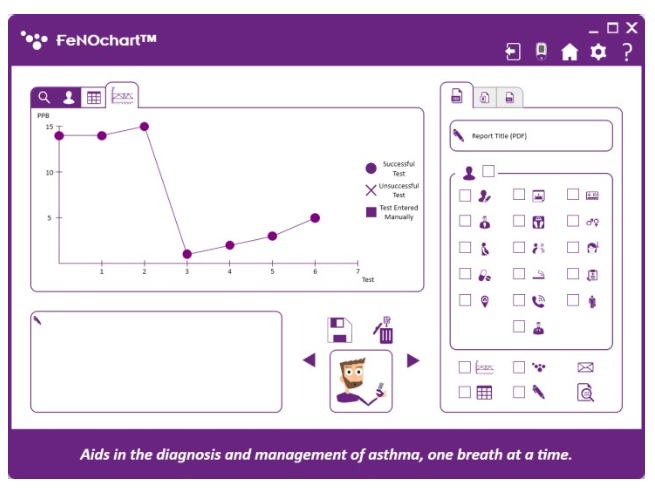
• View results in graph format with the ability to create reports in PDF format
• Automatic software updates
• Ability to remotely update the firmware of your NObreath®
Software Overview
NObreath® Technical Specifications
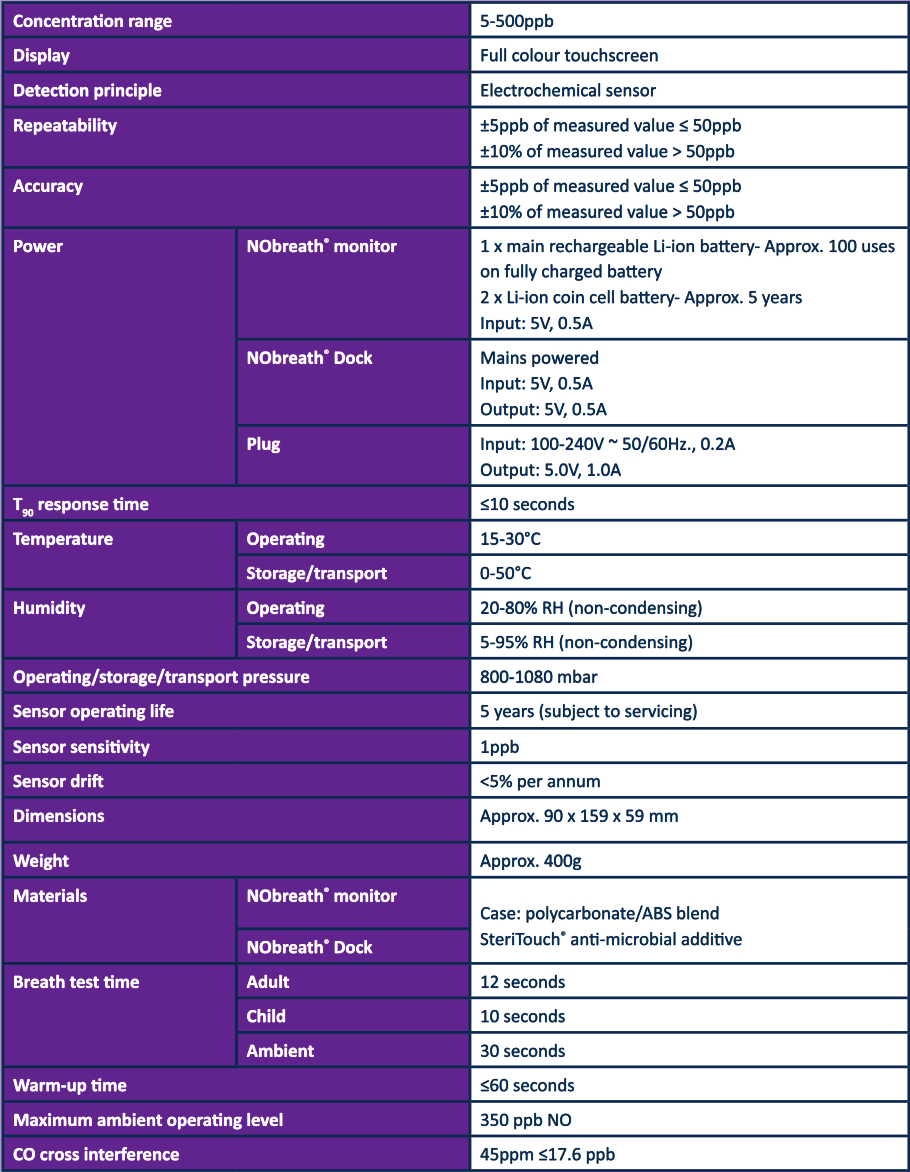
NObreath® Reference Materials
NObreath® Videos
What is FeNO?
What is the NObreath®?
NObreath® demo modes for patients
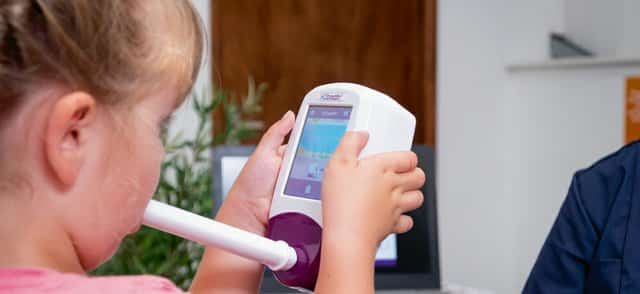
How to take a NObreath® FeNO breath test?
Intro to NObreath® FeNOchart Database Software
NObreath® Calibration
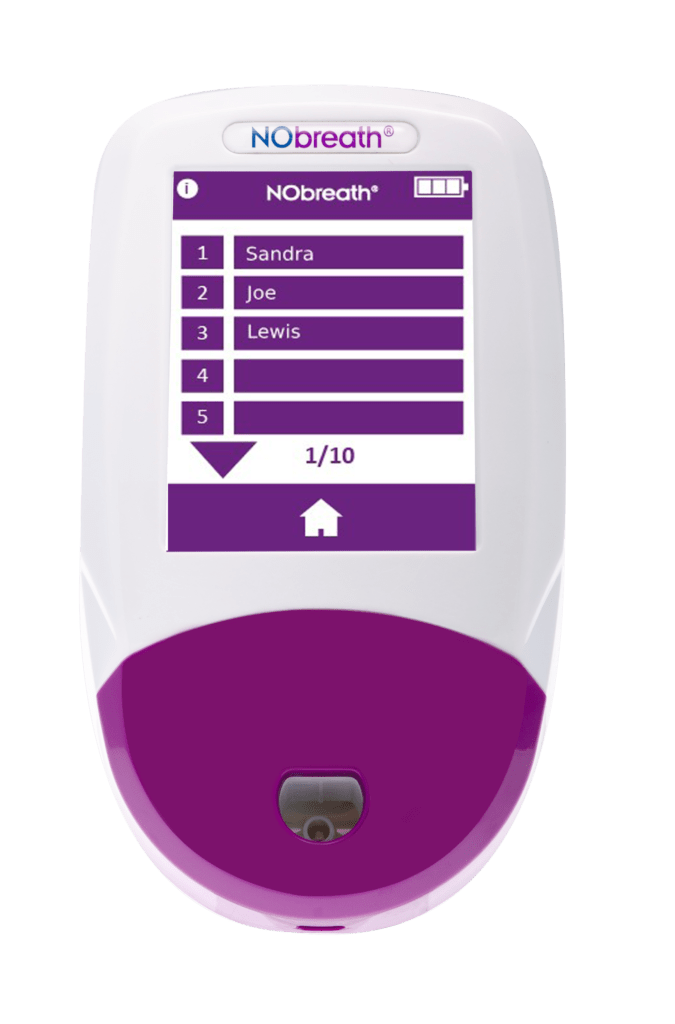
NObreath Patient Profiles
Charging the NObreath
What is FeNO?
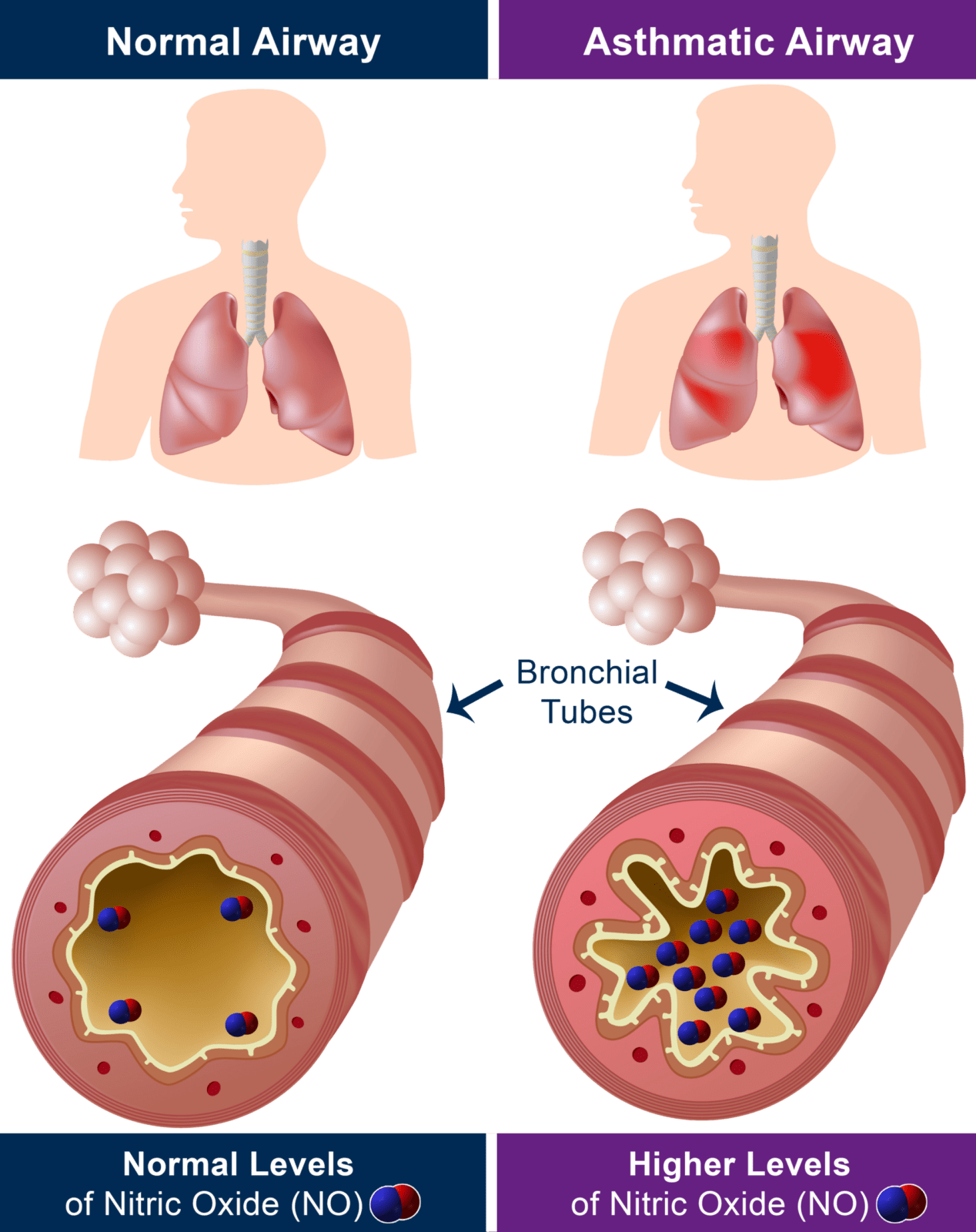
FeNO (fractional exhaled nitric oxide) are very miniscule particles of the gas nitric oxide (NO), measured in parts per billion. NO is naturally produced by your body to help combat inflammation and when your airway is inflamed, NO is produced in the lungs and exhaled on the breath.
The production of nitric oxide is often found to be higher in inflammatory conditions such as asthma and therefore FeNO monitoring can be used for the detection and management of such conditions2.
Airway inflammation is a central process in asthma and other lung diseases1. Being able to detect eosinophilic airway inflammation and monitor a patient’s response to treatment is regarded as a gold standard in the management of respiratory diseases.
Nitric oxide measurement is not intended as a stand-alone method for diagnosis and should be used in conjunction with other evaluation methods and tests3. Using FeNO measurements to evaluate airway inflammation in asthma represents a significant advance in respiratory medicine 4, but until now this has been an expensive test to deliver in everyday practice.
Benefits of FeNO Testing
- Non-invasive, quick, and easy to perform4
- Shows patient’s response to treatment, enabling the correct prescription of medication and safer/monitored adjustments
- Aids in identifying patients who do/do not require on-going treatment5
- Shows patient compliance
- Shown to be superior to the majority of conventional tests of lung function, such as peak flow recording and spirometry4
- Aids in differentiating between allergic (eosinophillic) and non-allergic asthma6
FeNO in Asthma
Asthma is commonly referred to as an inflammatory disease which affects a person’s airways, leading to hyper-responsiveness, obstruction, mucus hyper-production, and airway wall remodeling9. Although airway eosinophilic inflammation is a key characteristic of asthma, very few methods are available for measuring airway eosinophilic inflammation.
A majority of asthma patient’s airway inflammation is allergen driven Th2 response10. There is significant evidence from external literature suggesting FeNO (Nitric oxide) is a key biomarker tool for inflammation of the respiratory tract10. Increased levels of FeNO in asthma are thought to come from inducible NOS2 expressed in the inflamed airways10. The use of FeNO during asthma diagnosis and management, gives clinicians a more complete picture, helping them with key treatment decisions for asthma patients. The American Thoracic Society recommends the use of FeNO in addition to usual care for patients that are being considered for the treatment of asthma11. Please refer to the ATS website for the most up to date clinical practice guidelines on FeNO. Literature also suggests the ability for FeNO measurement to be used as good management tool for asthma12, assessing the effectiveness of treatments for patients, optimizing asthma treatments.
The Cost of Asthma
 USA: According to Asthma and Allergy Foundation of America (aafa), approximately 25 million Americans have asthma. This equals to about 1 in 13 Americans, including 8 percent of adults and 7 percent of children.13 It is the leading chronic disease in children.14 Currently, there are about 5.1 million children under the age of 18 with asthma.13 On average, ten Americans die from asthma each day. In 2019, 3,524 people died from asthma. Many of these deaths are avoidable with proper treatment and care.15 From 2008-2013, the annual economic cost of asthma was more than $81.9 billion – including medical costs and loss of work and school days: 16
USA: According to Asthma and Allergy Foundation of America (aafa), approximately 25 million Americans have asthma. This equals to about 1 in 13 Americans, including 8 percent of adults and 7 percent of children.13 It is the leading chronic disease in children.14 Currently, there are about 5.1 million children under the age of 18 with asthma.13 On average, ten Americans die from asthma each day. In 2019, 3,524 people died from asthma. Many of these deaths are avoidable with proper treatment and care.15 From 2008-2013, the annual economic cost of asthma was more than $81.9 billion – including medical costs and loss of work and school days: 16
- $3 billion in losses due to missed work and school days
- $29 billion due to asthma-related mortality, and
- $50.3 billion in medical costs
 UK: According to asthma UK, approximately 5.4 million people in the UK, receive treatment for asthma 8, and approximately 300 million individuals worldwide are thought to be affected by asthma9. It is thought that on average, 3 people die daily from asthma attacks in the UK.8 It is thought that the NHS spends approximately £1 billion a year treating and managing asthma symptoms 8.
UK: According to asthma UK, approximately 5.4 million people in the UK, receive treatment for asthma 8, and approximately 300 million individuals worldwide are thought to be affected by asthma9. It is thought that on average, 3 people die daily from asthma attacks in the UK.8 It is thought that the NHS spends approximately £1 billion a year treating and managing asthma symptoms 8.
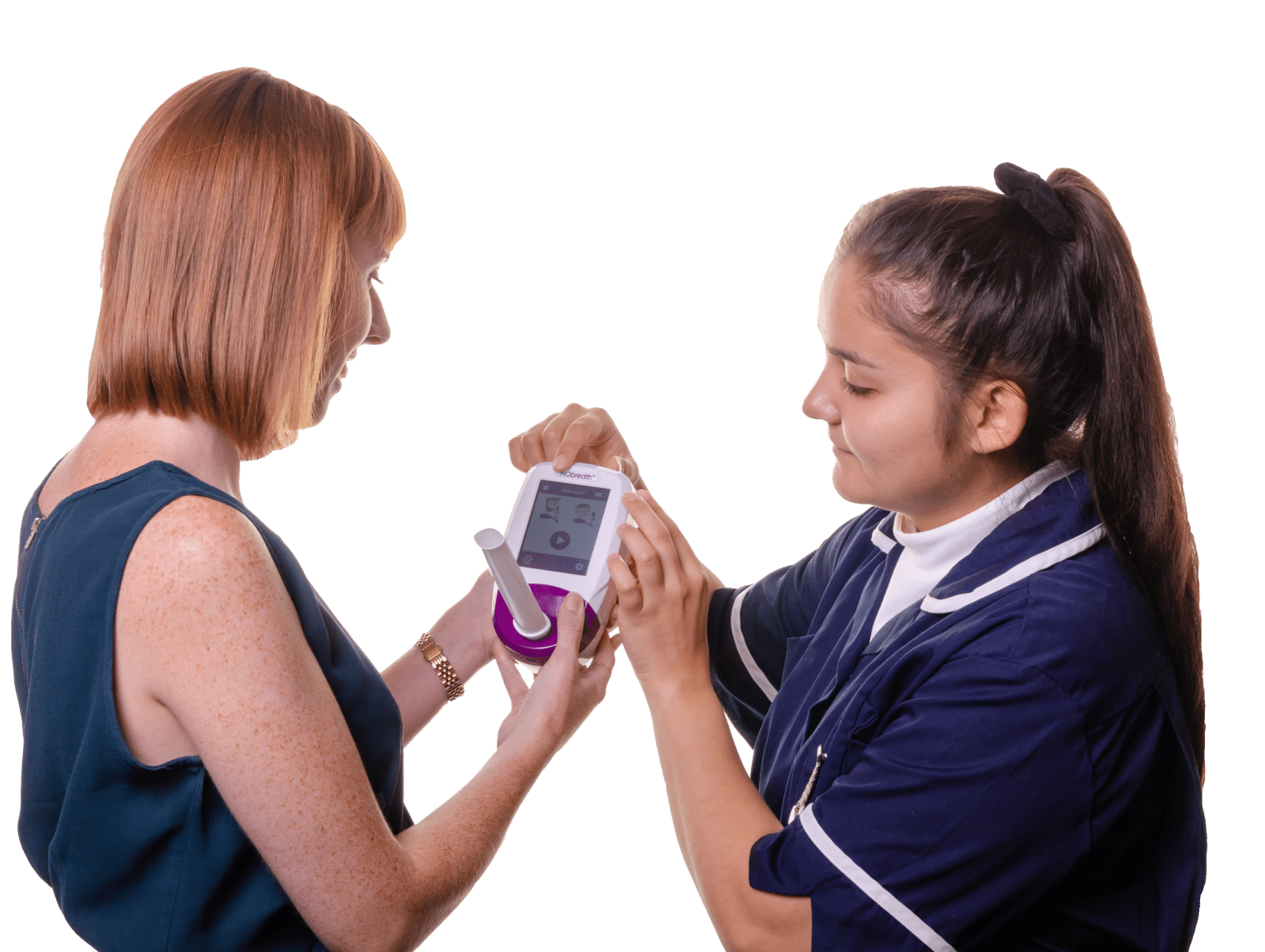
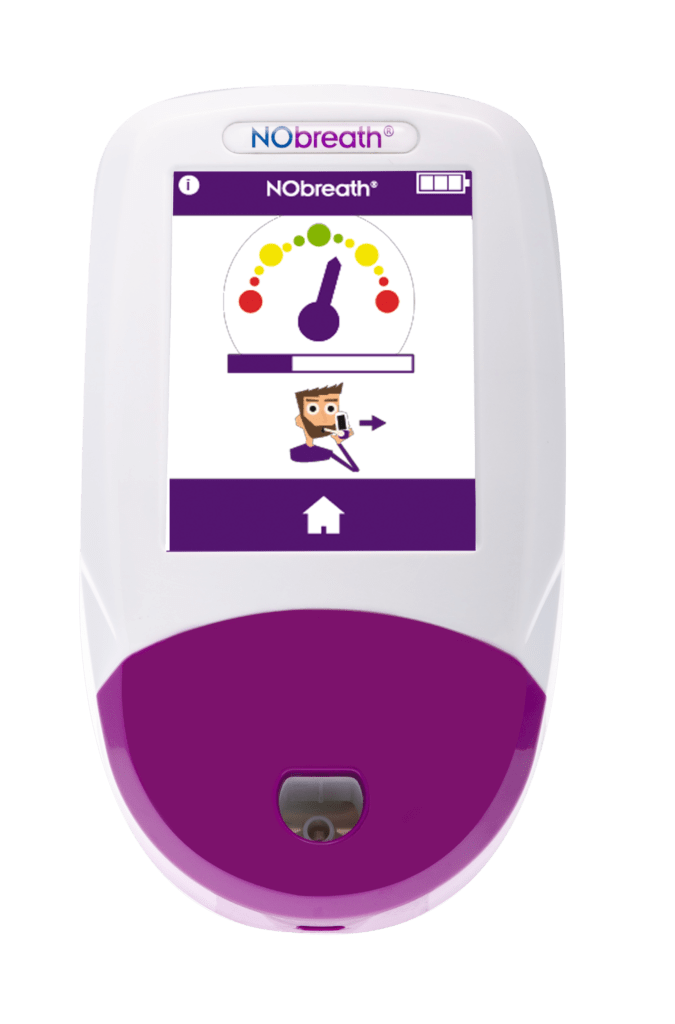
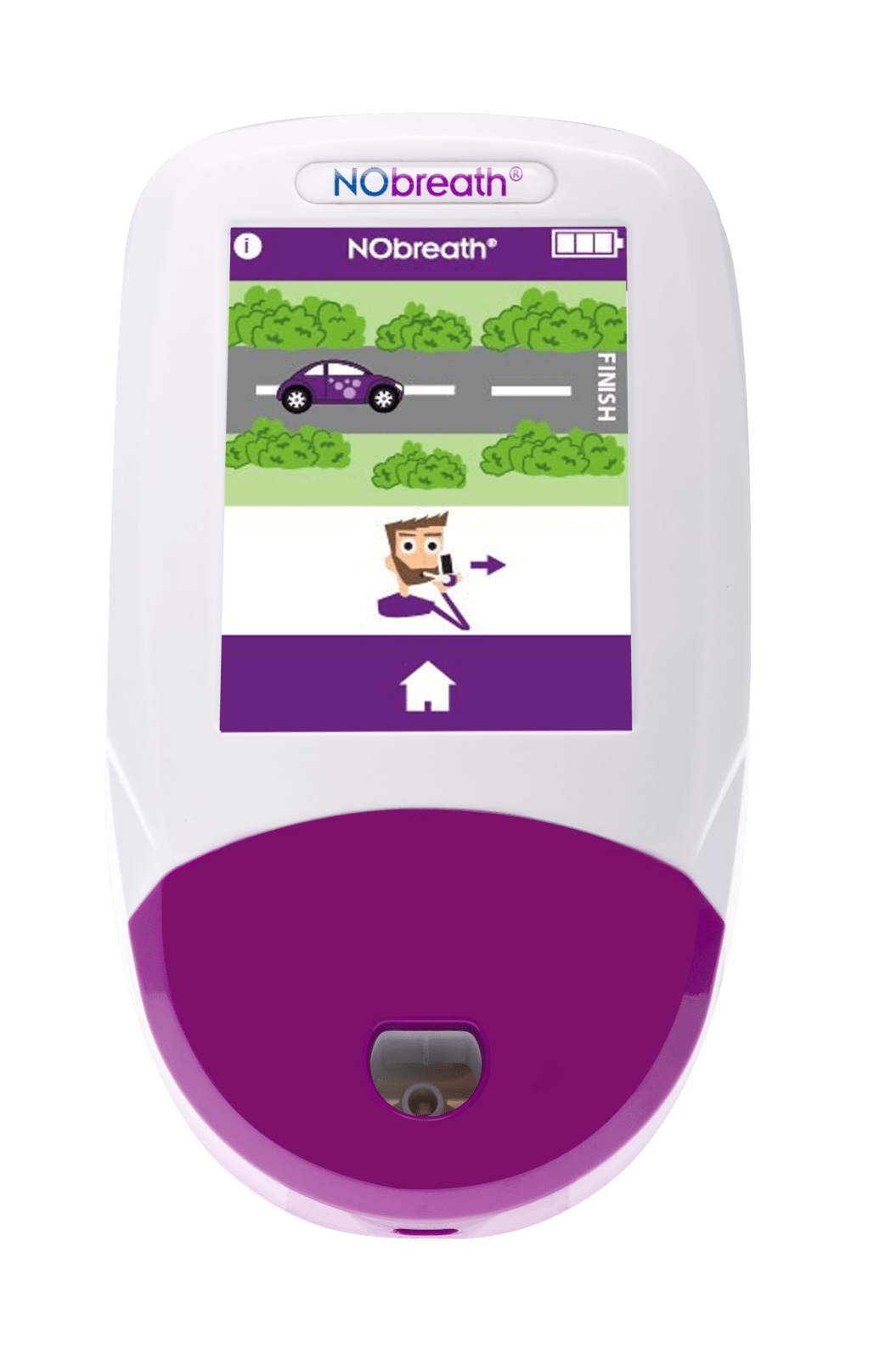
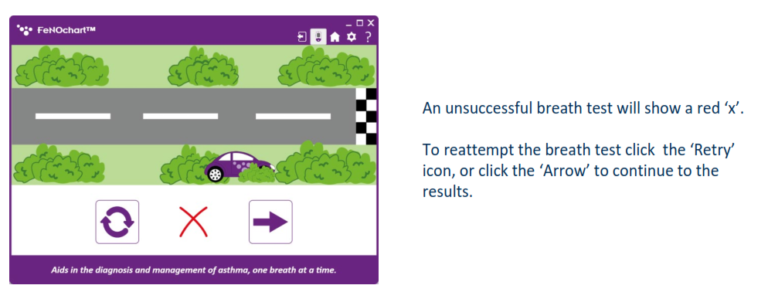
Simple Testing Process
Conducting a breath test is simple, non-invasive, and instant.


NObreath® Infection Control


NObreath® Mouthpiece Filtration Technology
The 2nd generation NObreath® Mouthpiece has an infection control filter, which has been tested vigorously by Public Health England to prove it removes and trap >99% and >98% of airborne bacteria and viruses respectively17.
The NObreath® mouthpiece has been tested to filter viruses as small as 23 nanometers in diameter and the COVID-19 virus particle has an approximate diameter of approximately 125 nanometres18 . Due to the risky nature of testing live respiratory viruses, a non-pathogenic virus model is used (M2-Coliphage). The filters undergo both bacterial filter efficiency (BFE) and viral filtration efficiency (VFE). The virus model is incredibly penetrable, even more so than a majority of human viruses, therefore makes it a very affective model to use for virus filtration efficiency (VFE) testing. The model virus is approximately 24-26 nanometers in size17 in comparison to COVID-19 virus which is approximately 125 nanometers in size 18. Therefore, Bedfont® can conclude that bacterial and viral pathogens (including COVID-19) will effectively be removed by both the NObreath® mouthpiece filter at an efficiency rate of >99% (Bacteria) and >98% (viruses)17.
The NObreath® Mouthpiece is a single-patient use mouthpiece, meaning it should be disposed of according to local waste guidelines immediately after testing to further minimize the risk of cross infection.
Furthermore, when taking a FeNO measurement with the NObreath®, due to our uniquely designed NO scrubber and software algorithms, the patient does not inhale through the device or mouthpiece before exhaling, to reduce the risk of cross-infection.
Additionally, the NObreath® Mouthpiece is individually wrapped to aid in handling and the highest infection control.

SteriTouch®
We are proud to say the NObreath® monitor is integrated with SteriTouch® antimicrobial additives, which eradicate the bacteria that cause contamination and infection. SteriTouch® has released a statement on COVID-19 which reads, “Several of the active substances used by SteriTouch® have been successfully tested against other enveloped viruses, such as Influenza, Avian flu and SARS. It would be reasonable to imply that those same active substances would be effective against COVID-19, but at this stage testing against COVID-19 is not available19.”
Why NObreath®?
The most features and benefits for your money.
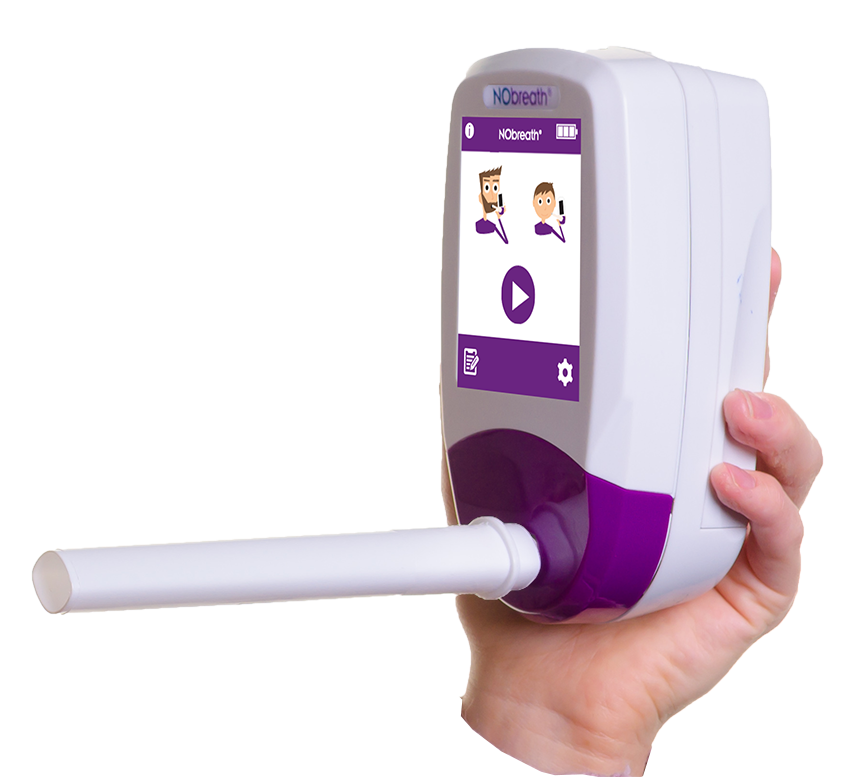
Key Features | NObreath®
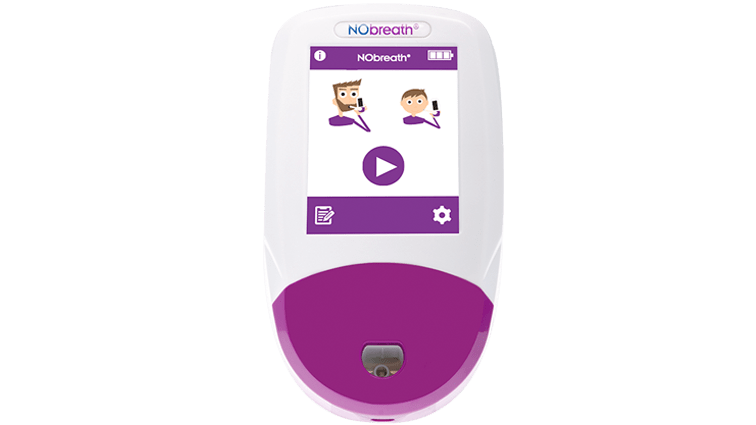
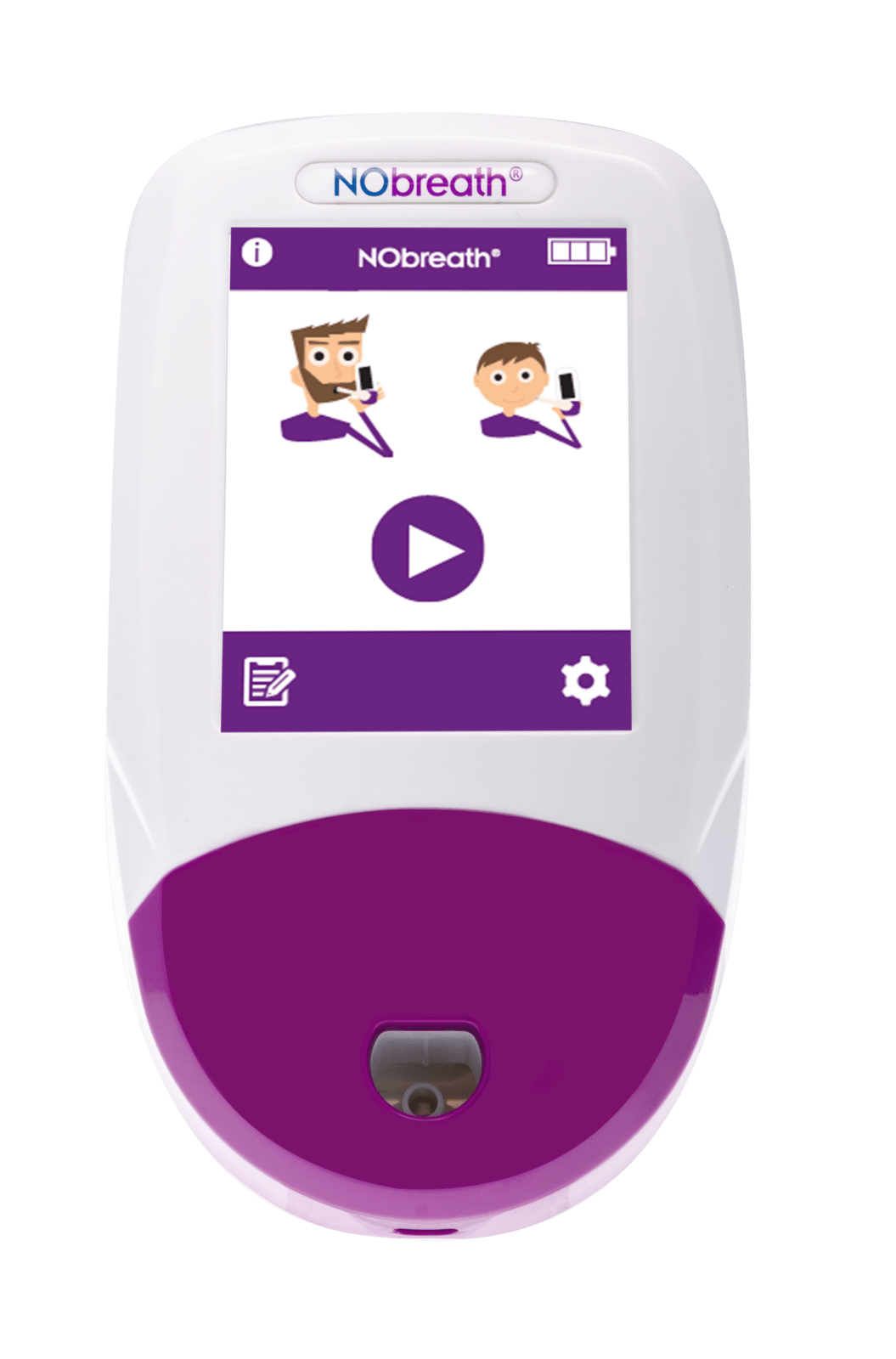
3 Test Modes



Interface Features
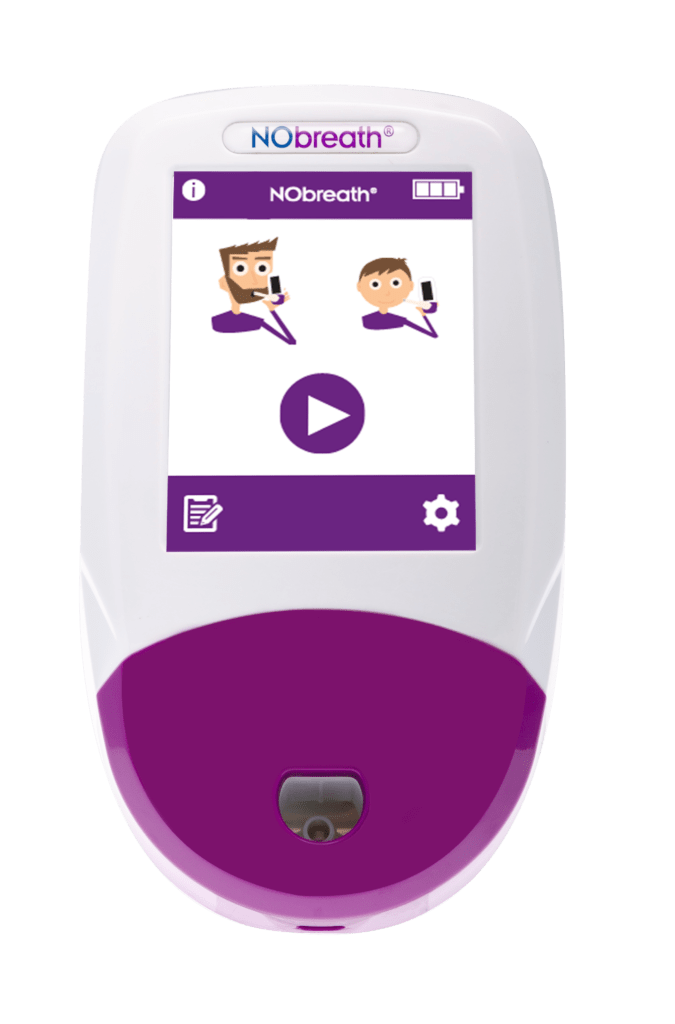
- Color Touch Screen - Simple and intuitive user interface
- Instant Boot Up < 60 seconds
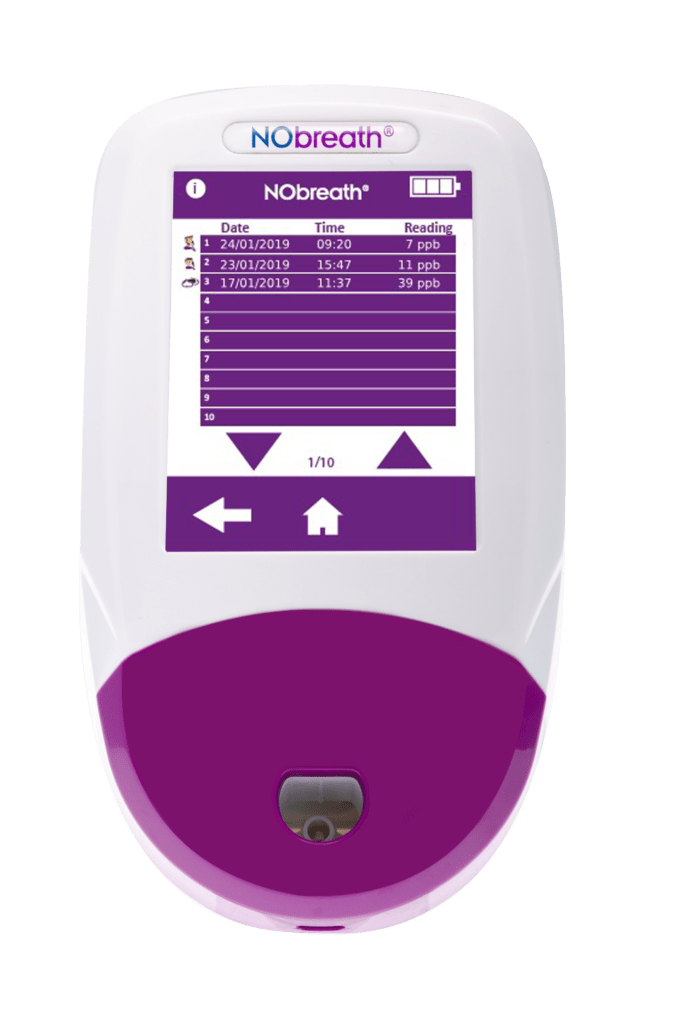
- Auto-Saves Readings - Automatically saves last 250 readings with time and date stamp
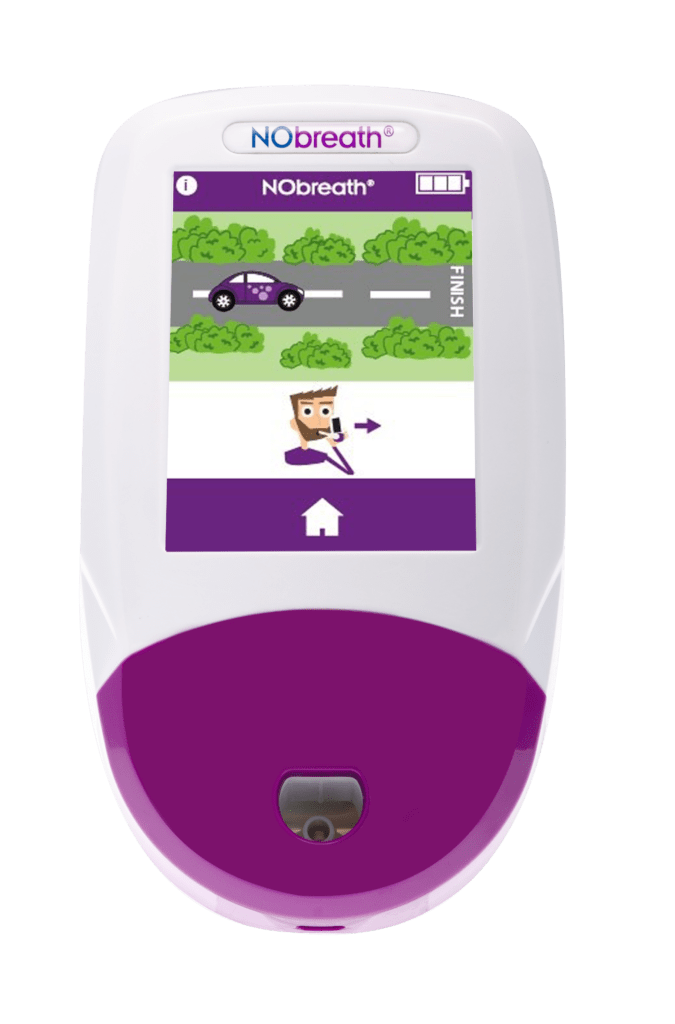
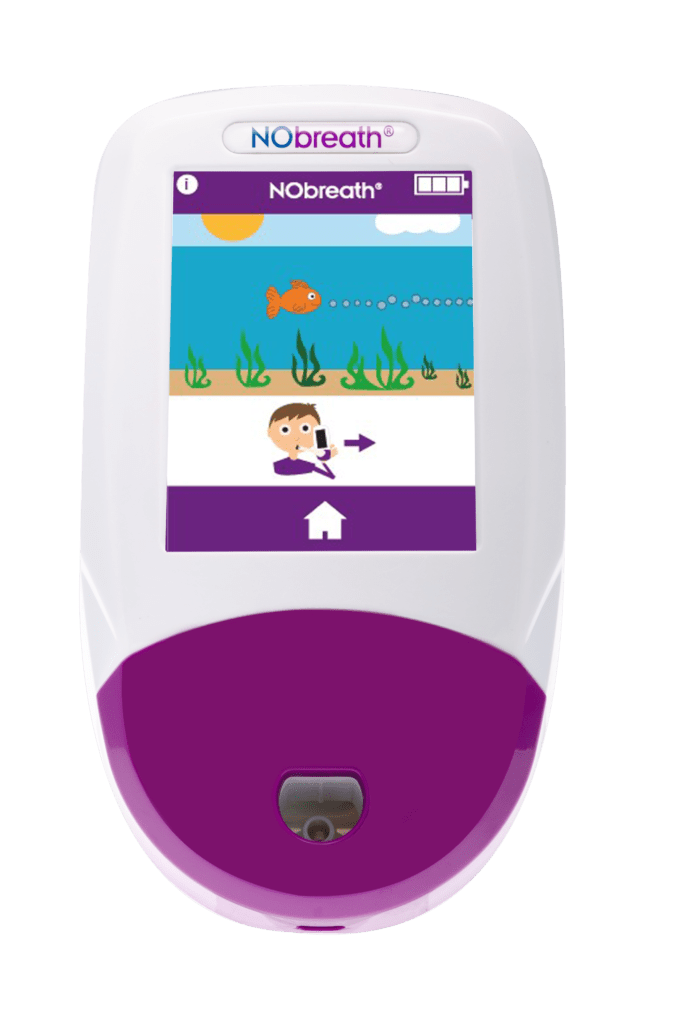
- Adult Exhalation Guide - Coaches patient through entire breath exhalation process to ensure proper technique and accurate results.
- Child Exhalation Guide - Coaches patient through entire breath exhalation process to ensure proper technique and accurate results.
- Patient Profiles - Ability to store up to 50 patient profiles with up to 25 results. **Pin Protected**
Reimbursement

AVERAGE REIMBURSEMENT FOR A
NObreath® BREATH FeNO Test
CPT Code: 95012 – Fractional Exhaled Nitric Oxide (FENO) measurement, is approved in the assessment of adult and pediatric beneficiaries with suspicion of asthma and for asthma management.
Contact us to learn more.
Ideal for the following industries
Scientific Literature
If you are interested in receiving supporting data, please contact us and we would be happy to search our literature archive.

Frequently Asked Questions
What is FeNo ?
FeNO (fractional exhaled nitric oxide) are very miniscule particles of the gas nitric oxide (NO), measured in parts per billion. NO is naturally produced by your body to help combat inflammation and when your airway is inflamed, NO is produced in the lungs and exhaled on the breath.
The production of nitric oxide is often found to be higher in inflammatory conditions such as asthma and therefore FeNO monitoring can be used for the detection and management of such conditions2.
Airway inflammation is a central process in asthma and other lung diseases1. Being able to detect eosinophilic airway inflammation and monitor a patient’s response to treatment is regarded as a gold standard in the management of respiratory diseases.
Nitric oxide measurement is not intended as a stand-alone method for diagnosis and should be used in conjunction with other evaluation methods and tests3. Using FeNO measurements to evaluate airway inflammation in asthma represents a significant advance in respiratory medicine 4, but until now this has been an expensive test to deliver in everyday practice.
What are the benefits of monitoring FeNO with the NObreath®?
- Non-invasive, quick, and easy to perform4
- Shows patient’s response to treatment, enabling the correct prescription of medication and safer/monitored adjustments
- Aids in identifying patients who do/do not require on-going treatment5
- Shows patient compliance
- Shown to be superior to the majority of conventional tests of lung function, such as peak flow recording and spirometry4
- Aids in differentiating between allergic (eosinophillic) and non-allergic asthma6.
What is the NObreath® Forum?
Included at no additional fee with the purchase of a NObreath®, you are eligible to become a member of the NObreath® forum – a place where you can seek answers, offer help or simply discuss FeNO techniques, findings and much more with other healthcare professionals from around the world. Contact us to learn more.
RECOMMENDED
NObreath® Accessories
-
NObreath® Mouthpieces
$300.00 -
Sale!
Cleansing & Antimicrobial Wipes
$7.50 -
Alcohol Free Hand Sanitizer
$15.00







































Do you bill insurances for medical services?